Initial disease classification within acute leukemia relies on identifying morphological and immunophenotypic features of hematopoietic differentiation retained by leukemic blasts. While existing approaches can classify leukemic cells into broad lineages, they lack precision in discerning between specific cell states, particularly at the level of immature blasts. Single-cell (sc) RNA-sequencing (RNA-seq) provides thousands of new markers to enable precise determination of leukemia cell state and provides an opportunity to refine our classification of acute leukemia.
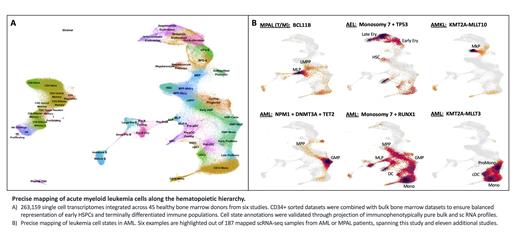
To extend our ability to identify leukemia cell states by scRNA-seq, we developed a comprehensive reference map of human bone marrow hematopoiesis. Specifically, we integrated three unsorted bone marrow datasets and three CD34+ hematopoietic stem and progenitor (HSPC) purified datasets to ensure balanced representation of early HSPCs and terminally differentiated immune cells. This culminated in a curated atlas of 263,159 high-quality sc transcriptomes, representing 55 cellular states spanning 45 healthy donors (Fig 1A). We validated our cell state annotations to ensure concordance between transcriptional states and functional populations through reference mapping of bulk and sc transcriptomes from purified HSPC subsets. For example, mapping of scRNA-seq profiles from Lin-CD34+38-45RA-90+49f+ long-term (LT) HSCs revealed 91% concordance between transcriptional HSC and functional LT-HSC.
We next performed scRNA-seq profiling on 12 AML patient samples harbouring either myelodysplasia-related genetic features or classical alterations involving NPM1 or KMT2A. By mapping leukemia cells onto our reference atlas, we identified pronounced involvement of early erythroid-like blasts in a subset of AML samples. To further investigate unconventional lineage priming in AML, we processed and mapped scRNA-seq profiles from eleven studies comprising 166 additional AML samples and 9 mixed phenotype acute leukemia (MPAL) samples (Fig 1B). After quality control and exclusion of mature lymphoid cells, we performed composition analysis utilizing 600,570 leukemia cells mapped to 38 cell states to identify patterns of variation among leukemia cell states. Immature leukemia cells map to a range of cellular states spanning HSC/MPP-like, LMPP-like, MLP-like, GMP-like, MEP-like, and EarlyEry-like, among others. Notably, we identified a subset of AMLs with high MLP-like enrichment that co-cluster with MPALs as well as a subset with high EarlyEry-like involvement that co-cluster with acute erythroid leukemias (AEL), thus highlighting the biological continuum between these diseases.
We next identified marker genes from each leukemia cell state and trained sparse regression models to estimate their relative abundance within bulk RNA-seq cohorts. Through bulk analysis, we confirmed that MPAL is highly enriched for MLP-like cells compared to AML (p=2.5e-12) and that M6 AELs are highly enriched for EarlyEry-like cells compared to M0-M5 AMLs (p=0.00067). Among 864 AML patients, we found that high MLP-like abundance was associated with NUP98-NSD1 fusions (p=0.0011) and RUNX1 mutations (p=8.0e-10) while high EarlyEry-like abundance was associated with Complex Cytogenetics (p=8e-15) and TP53 mutations (p=2.3e-25). Cellular states of immature leukemic cells captured inter-patient heterogeneity within 136 AEL samples, wherein high EarlyEry-like abundance was associated with TP53 mutations (p=3.6e-9) and Poor cytogenetic risk (p=1.1e-9) while high GMP-like abundance was associated with KMT2A alterations (p=0.00046) and Good/Intermediate risk (p=0.00032).
Finally, through integration of sc transcriptomics and targeted DNA profiling, we found examples of genetic subclones impacting the cellular hierarchies of individual patients through induction of early differentiation blocks, skewing of lineage output from myeloid to erythroid, or potentiation of transcriptional self-renewal programs within mature myeloid cells. Together, our high-resolution mapping approach highlights the cellular state continuum between acute leukemias and enumerates the impact of genetic drivers on leukemia cell hierarchies. Precise definitions of cellular states, coupled with the presence or absence of genetic alterations, may help to refine future disease classification, prognostication, and therapy development.
Disclosures
Haferlach:MLL Munich Leukemia Laboratory: Current Employment, Other: Equity Ownership. Mullighan:Pfizer: Research Funding; Abbvie: Research Funding; Amgen: Honoraria; Illumina: Honoraria. Dick:Trillium Therapeutics Inc/Pfizer: Patents & Royalties: Trillium Therapeutics; Graphite Bio: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene/BMS.: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal